 Margarete Ertl, Germany
Margarete Ertl, Germany
Conductor and vocal coach
This article is based on a lecture which would have been part of the WSCM 22 in Auckland, New Zealand. As the Symposium had to be cancelled, ICB offers you here a possibility to take a glimpse into the subject through a written version.
Look into the fascinating world of neuroscience and get an insight into the neuronal symphony. The first perceptions of “musical elements” already stimulate the prenatal human brain. With each musical perception, rehearsal and concert, conductors, composers or singers work on their personal neuronal network.
While singing, the human brain processes more than verbal and musical syntax. It controls involuntary body functions, movement of the vocal tract, the eyes, limbs or posture. It corrects and fine-tunes the muscles through sensory, visual or auditory input. At the same time it memorizes, thinks ahead, calculates, finds solutions, keeps focused, and processes visual signs from score and conductor. It constantly controls breathing and voice to pitch, timbre, beat, being in time, rhythm, spectral properties of vowels and consonants in various languages. It learns and remembers the comments and demands relating to the expression of the music. The singer is interacting with other musicians, not only musically but also emotionally and socially, blending with the other voices and keeping in mind the acoustic requirements. The ear is permanently alert, focused on the singer’s own vocal production and all other surrounding sounds while also analysing sounds in a hierarchical order of attention. In addition to all this, chemical processes simultaneously guide personal sensitivities and emotions. To accomplish this proficiency a highly complex neuronal network is required.
This article summarizes the current status of research findings focused on the field of singing, a scintillating journey into the magic of music. You will have a view into the human brain and its nervous system first. This report furthermore introduces the functional neuroimaging techniques that map the activities in the different structures of the human brain during complex vocal processing. The final topic pictures the brain’s anatomy and opens a window into enthralling subject areas of recent research. It will display neuronal and interactional processes in the various brain regions thatwhich constitute singing according to the findings of renowned researchers.
Neuroscientific research decodes step by step neuronal processing with state-of-the-art diagnostics and this reveals fascinating details of the neuronal symphony in our active brain while singing, imagining, and listening to music. For a long time the focus of neuronal research into music processing was on instrumentalists. But in recent years, researchers have discovered the singing voice with its great complexity as an object of increased research interest.
About 80 billion neurons are communicating with each other incessantly. Electric impulses and chemical processes interact with gray matter (neurons and glia cells) and white matter (myelinated axons). The cerebral structure-density increases with electrical or chemical excitation or degenerates when not being used. The electric impulses can be made audible by means of »patch clamp technique«. Thus scientists can both collect data and listen to the neuronal symphony in real time. Our brain is not only processing information but it is, somehow, music itself or is »singing« our thoughts into existence.
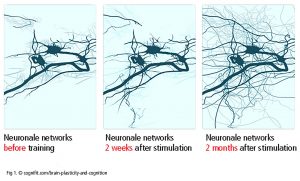
Neurons regulate everything, from the autonomic nervous system to cognition and guide a large number of networks between brain areas (Luo et al., 2019). The communication between neurons at the synapses is referred to as synaptic plasticity[1]. Neurons, axons and glia cells communicate with other neurons at the point of origin or in the target area (neuronal plasticity – Fig 1). One neuron can mesh with up to 10 000 other neurons, up to 1000 Terabytes capacity totally (Bartol et al., 2015). In addition to this asterozytes and microglia are involved in repair processes and act as an intermediary in exchanging information between neurons. Synaptic and neuronal plasticity is well documented. It is not conclusively clarified if the adult human brain is able to grow new neurons (’neurogenesis’ in subventriculare zone, striatum and hippocampus).
Korbinian Brodmann was a pioneer in mapping the human brain. In 1909 he recognized 52 different histologic areas in the brain (Brodmann Areas – BA), but he was able to study pathological brains only. Today’s modern functional neuroimaging techniques enable researchers to look into the active living brain. Two prominent participants in imaging studies were Herbert von Karajan and Rénee Fleming. As long ago as the 1970s Karajan made available several opportunities to help researchers to collect data while he was conducting (Müller, 2005). In 2017 Fleming was fascinated to be part of an fMRI study to discover what is happening in the brain network while singing at a professional level takes place.
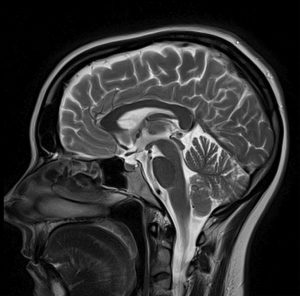
According to the setup of the studies, the collected data of the chosen imaging technique were converted, rendered and colored into breathtaking pictures showing the regions of interest (ROI). Electroencephalography (EEG) and Magnetoencephalography (MEG) are the most commonly used non-invasive imaging techniques to study regions which show activity in grey matter during singing. The EEG reaches structures on the surface of the brain only and has a low spatial resolution. However, it detects changes in electrical activity in real time, nearly on a millisecond-level. The MEG, with resolution similar to EEG, is used to measure the magnetic fields produced by cerebral electrical activity.
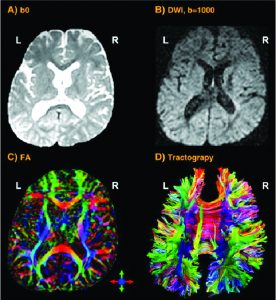
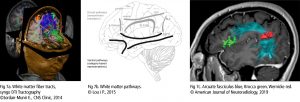
If the research is focused on the cortical thickness of the grey matter or the size or tracts of white matter fibers, imaging techniques with a higher spatial resolution are necessary. Magnetic Resonance Imaging (MRI – Fig 2a) and the functional MRI (fMRI) are frequently used to approach this. They visualise the anatomical structures of the brain, even in subcortical areas. The method detects the changes in blood oxygenation and blood flow which occur in response to neural activity. The Blood-Oxygen-Level Dependent (BOLD) is a specific method of fMRI, which uses the different magnetic properties of deoxygenated and oxygenated haemoglobin to measure voice activity in related brain areas.
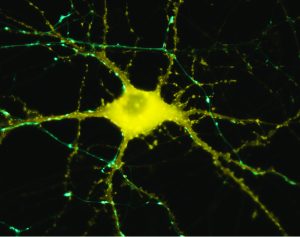
Microscop image of neuron in response to stimulation
Increased excitation activates olygodendrocytes to mould a myelin sheath around the axon (nerve fiber). Thus the action potential changes from continuous propagation along the axon, to saltatory propagation from one non myelinated area (node of Ranvier) to the next. This speeds up the transmission of electrical impulses. One oligodendrocyte can myelinate up to 50 neurons in the process of learning or when restoring broken connections. Oligodendrocytes can also demyelinate axons to extinguish information and clear up connections. The Diffusion Tensor Imaging (DTI – Fig 2b) detects fibers in magnificent detail. Like MRI and fMRI, DTI is non invasive and uses the unique directional movement of water molecules along the neural tracts to identify the linkages and structures of white matter fibers with low temporal resolution.
Further non invasive tools to collect promising data in vocal studies are Magnetic Resonance Spectroscopy (measuring biochemical processes in a strong magnetic field) or 3D high resolution microscope imaging techniques (Fig 2c). They allow precise scanning a specimen in nanometer and subnanometer range. Single Photon Emission Computed Tomography (SPECT) and Positron Emission Tomography (PET) use short-lived radioactive material. Despite their low resolution, both methods map functional processes effectively by measuring blood flow. Areas of high radioactivity are associated with high brain activity in the subcortical regions.
Each imaging method has its advantages and disadvantages. Summarizing the results of each different approach and the ROI, increases our understanding of neuronal processing while singing.
The main anatomical characteristic of the human brain consists of the folded structure of the cortex composed of two hemispheres connected by a corpus callosum. The different imaging techniques verify lateralization. This means that the two hemispheres have areas which are involved in processing different tasks. Some are oriented more to the left or the right, orthey may be equilibrated. The left hemisphere is specialized in rapid, the right hemisphere in slow temporal processing (Poeppel, 2003).
Song and speech areas are well-known examples of lateralisation. They switch between left and right language processing areas and also share them. Language production and some aspects of syntactic processing are localized primarily in areas of the left anterior hemisphere, including Broca’s area (BA44/45). Language comprehension is confined primarily to the left posterior temporal–parietal region, including Wernicke’s area (BA22). The left planum temporale is up to ten times larger in the human brain than its right homologue (sensitive area for pitch perception as part of BA22). Broca’s area shows larger volume in the right hemisphere. Also the central sulcus, housing the primary motor cortex (M1- vocal tract), is reported to be deeper and larger in the right hemisphere. (Corballis, 2014; Toga et al., 2003; Albouy et al., 2020).
© Butler B., 2013
An unexpected finding in research is a thicker cortex or white fiber bundle; however, this does not imply an overall advantage per se when comparing non-musicians to musicians. After long-term practicing the brain areas are used more efficiently and the fine-tuning of the muscles in the vocal processing is producing high sensory feedback. This can be measured in some areas as a thicker physical stucture, but not implicitly. A net of synapses with higher density per neuron and increased glial cell density are assumed to be involved in this more effective processing (Gaser, Schlaug, 2003).
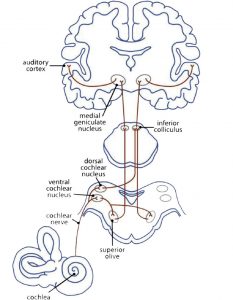
At first sight music is connected with the sense of hearing. The myelinisation of the axons in the auditory cortex already begins in the mother’s womb during the last trimester of pregnancy. The neuronal auditory network (Fig 3a) starts with a mechanical impulse that reaches the eardrum. The fluid in the cochlea passes the impulse on to the outer and inner haircells in the corti organ, the stereocillias. These change their position and give an impulse to their root cells. Chemical transmitters pour into the synaptic gap to open Ca2+ channels. This brings about an electric impulse to the auditory pathway: from the cochlear nuclei to the superior olive, inferior colliculus, and medial geniculate nucleus (thalamus) to the primary auditory cortex (A1) in the superior temporal gyrus (STG). Here the impulse is passed on to networks that analyse the information of the sound (Cheung et al., 2017).
Heschl’s gyrus (HG) corresponds to the auditory cortex (Fig 3b). It is closely involved in sound perception and its morphology in shape, size, and asymmetry also influences musical performance. A larger right HG indicates a preference in spectral pitch processing, where sound is broken up into its different components. Preference in fundamental pitch to perceive sound as a whole gives rise to a larger left HG (Brenner, Schneider et al., 2017). For instrumentalists, this is well documented. Further investigation is needed to examine the difference in spectral vs fundamental pitch processing in HG with singers and how far this may influence the sound of a choir.
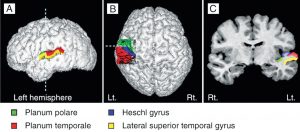
The brain structures of singers and instrumentalists are significantly different. The singers‘ instrument, the voice, is part of their bodies. It is steadily growing and aging and is permanently used in speech and song. Singers rely not only on auditory but also on sensorimotor feedback during the process of vocal production. Based on increasing singing expertise through lifetime learning, they show thicker grey matter in the auditory (f.e. HG) motor areas (f.e. supplementary motor area – SMA) or the cerebellum. Also, the white matter in commissural fibers or association fibers is of higher density, and the volume in the left hemisphere is larger than in instrumentalists. (Loui, 2015; Schlaug et al., 2011).
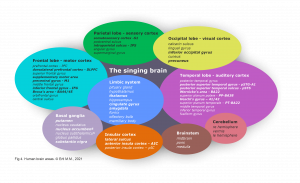
The cerebral cortex comprises the frontal, temporal, parietal, and occipital lobes with its twenty-seven sulci and sixteen gyri, cerebellum, and brain stem (Fig 4). The cerebral cortex covers further internal structures: these include limbic system, basal ganglia, and the white fibers. Commissural fibers (corpus callosum, fornix) conduct the information transfer and connection between the hemispheres. Association fibers (arcuate fasciculus/ longitudinal fasciculus/ uncinate fasciculus) connect brain regions along and deep within the same hemisphere. Projection fibers are the connection to the spinal cord. These structures respond to singing directly or secondarily.
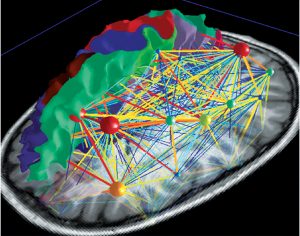
Deliberate control over vocal motor production for speech and singing activates a highly complex network which is hierarchically organised and can be visualised as a map (Fig 5a). There are main hubs connecting nodes with different levels of significance. So the neuronal network is growing into a personal and absolutely individual brain map (Fig 5b). It is quite challenging to obtain a comprehensive picture of this complex network, but it is breathtaking to detangle one sector back-to-back (atlas.brainnetome.org/bnatlas.html).
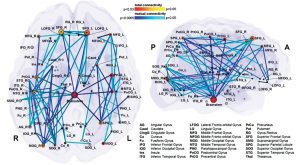
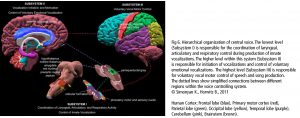
The central network in singing is the hierarchically organized vocal control network. The anterior cingulate cortex (ACC – an important hub in music processing), inferior parietal and occipital lobe, part of the limbic system and the periaqueductal grey (PAG) in the midbrain of the brainstem (Fig 6) are the crucial areas. They pass the information on to the phonatory motoneurons attributed with the readiness or initiation of vocalization (Jürgens, 2009). These regions are connected to the network which is generally attributed to learning vocalization, consisting of M1 in the frontal lobe and regions that fine-tune the vocal program from the motor cortex: putamen and globus pallidus, pontine gray (pons), and cerebellum. The tuned program is sent back to M1 via the thalamus. In addition to this, there are various neural regions of the auditory pathway involved (as described above).During pitch comparison in single notes or melodies, a recruitment of cortical areas within and outside the temporal lobe in auditory and frontal lobe areas are engaged. The vocal timbre is processed in the superior temporal sulcus (STS) within the temporal lobe: the anterior STS (aSTS – voice recognition), midanterior STS (spectralcharakteristic of voice, comparison sound to noise), the posterior STS (pSTS – identify unfamiliar voices). The somatosensory circuit with intraparietal sulcus (IPS) and dorsal premotor cortex prepares sensory input to motor preparation. The feedback to the sense of movement and position (respiratory/ laryngeal) is processed in the primary somatosensory cortex (S1) of the parietal lobe and the insula. (Kleber, Zarate, 2014). ACC, pSTS, IPS, AIC and cerebellum interact with the motorcortical control of complex vocalization.
The anterior insula cortex (AIC) and cerebellum are important hubs for voice processing and are to be considered in more detail. The AIC integrates inputs of the auditory, somatosensory, visual, and motor regions. Its crucial role in singing occurs in the larynx (left dorsalAIC) and the diaphragm (bilateral dAIC) together with the thalamus (bilateral postrerior/left dAIC) and the left putamen (left dAIC) (Zamorano et al., 2019). The cerebellum participates especially in timing and fine regulation of motor control (larynx, facial muscles, balance, eye movement, coordination precision). It is also found active in cognitive functions and emotional control (serotonin and norepinephrin pathways). Vocal-skill learning causes structural change to grey matter density in primary and secondary somatosensory cortex and IPS in relation to experience. This plays an essential role in singing skill development.
In addition to grey matter activity, scientists discovered that white matter fibers (Fig 7a) take an important role in connecting the areas concerning the voice. The dorsal pathway (Fig 7b) for sensimotor translation is described as flexible and indirect. The premotor cortex (PMC), supplementary motor area (SMA), and pre-SMA interconnect via u-fibers (pitch, sequencing grammar) and the superior longitudinal fasciculus (focus attention on visual space, role in the articulatory components of language). The arcuate fasciculus connects temporal and frontal brain regions, STG with PMC and IFG (among others BA44/45 with BA22 – Fig 7c). They are involved in auditory and vocal output, feedback from the larynx (singing accuracy) and lungs in response to vocal training.
The ventral pathway (Fig 7b) is involved in more automatic and direct processing. The inferior longitudinal fasciculus with the extreme capsule fiber system connects the middle temporal gyrus with BA44/45/47 and IFG in lexical semantic information, pitch and melody analysis. The uncinate fasciculus links orbifrontal cortex (OFC) to anterior temporal lobe for voice processing, memory, emotion and decision making. The inferior longitudinal fasciculus merges occipital lobe and temporal lobe to identify visual objects (Bashwiner, 2018).
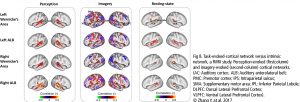
The Default Mode Network (DMN) and Imagery are two significant networks correlated to singing. The DMN is active during wakeful rest or mind-wandering. It is connected to musical creativity as in improvising, making or writing music. Imagery interacts with the DMN, too. It is an amazing skill, to reflect music only mentally, wholly focused on the related tasks. It combines i.a. auditory processing (f.e. bilateral BA22), sensorimotor coordination, memory retrieval (cerebellum), cognitive control (PFC), visual areas, and emotion (limbic system). As in vocal production, imagery is an interaction between cortical areas and multiple networks, including auditory, attention, and motor-control networks. (Fig 8. – Zhang et al., 2017). The DMN links the dorsomedial PFC, lateral temporal cortex and temporal pole (BA38), posterior CC, precuneus and angular gyrus as well as the inferior parietal lobe. Together with hubs of motor activity and sound processing (like dorsal PMC, SMA and BA22) and emotion-related areas (OFC, BA38 and amygdala), the DMN forms a highly creative workstream.
Beneath the anatomical network structures, neurochemical processes interrelate with vocal processing. The brainstem, basal ganglia and limbic system produce neurotransmitters and hormones, which communicate with neurons in the target brain areas (striatum, nucleus accumbens, ACC, PFC). They inhibit or exhibit activity via excitatory glutamatergic pathways, inhibitory GABAergic pathways, and dopaminergic pathways. The cerebellum and amygdala are important parts of serotonin and norepinephrine pathways to stress response as reward.
Dopamine and opoids (ventral tegmental area, substantia nigra) play a crucial role in reward-related behaviors like motivation, pleasure, attentional control, and they also reinforce learning. Cortisol (pituitary gland) and serotonin (raphe nuclei) are shown to control stress and arousal. They initiate or subdue stressors to reconcile cognitive function, emotion, and unwitting body functions. Serotonin boosts the immune system, relaxes, has an antidepressive effect, and promotes motivation. Oxytocin (pituitary gland) and endogenous opoids such as dopamine and vasopressin regulate immunitiy, pleasure sensations, and social affiliation, so singing in a choir presumably mediates social effects. The exact role of oxytocin in singing is not yet understood in detail. (Chanda, Levitin, 2013).
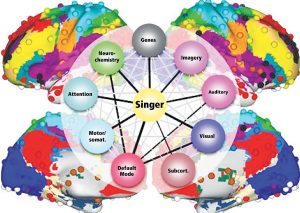
To complete the picture, the research field of our genes has to be mentioned briefly. Scientists found promising data in linkage between musical aptitude and chromosomes (Tan et al. 2014; Oikkonen et al. 2015). Genes in chromosomes are responsible for producing proteins or receptors which coalesce to neurotransmitters. Through these, the linkage to the brain areas was detected. Here are some findings: gene GATA2 in chromosome 3q.21.3 regulates the development of cochlea hair cells and inferior colliculus in the auditory pathway. Multiple genes in chromosome 4 point out involvement in song learning, memory, and musical perception and are associated with singing accuracy. Genes in chromosome 8 are connected with learning and memory, absolute pitch and musical perception. Gene SLC6A4 in chromosome 17q11.2 is associated with reward seeking, musical memory, and group activity like choral singing or dancing. It is stunning to monitor gene positions in chromosomes in the human dat base gene cards (f.e. genecards.org) with regard to their contribution to singing.
More regions in the whole brain are involved and activated bilaterally especially by singing than was previously believed. Multiple neuronal networks in grey and white matter intertwine with chemical pathways and genetic predisposition. Like cog-wheels in a huge clockwork machinery they create a neuronal symphony. Neuroscience shows the beauty of the human brain and its amazing capabilities. The results of this exciting research field could help to treat impairment of the vocal tract. Promising studies explore the recovery of the voice through artificial, 3D-printed vocal tracts, using individual image data. This is an encouraging begining for further investigation (Howard, 2020).
However, there is still a great deal to discover about the process of singing. Because the more answers we find, the more questions arise, and at the same time our fascination with the human brain and the magic of music grows.
References
- Albouy P., Benjamin L., Morillon, Zatorre R.J., 2020. Distinct sensitivity to spectrotemporal modulation supports brain asymmetry for speech and melody. McGill University, Montreal, Canada. doi:10.1126/science.aaz3468
- Alexander A.L., Lee J.E., Lazar M., Field A.S., 2007. Diffusion Tensor Imaging of the Brain. University of Wisconsin-Madison; New York University School of Medicine, New York USA., doi:10.1016/j.nurt.2007.05.011
- Altenmüller E., Furuya S., 2017. Apollos Gift and Curse: Making Music as a model for Adaptive and Maladaptive Plasticity. Hochschule für Musik, Theater und Medien Hannover, Germany. doi:10.1515/nf-2016-A054
- Bartol Jr. T.M., Bromer C., Kinney J., Chirillo M.A., Bourne J. N., Harris K. M., Sejnowski T.J., 2015. Nanoconnectomic upper bound on the variability of synaptic plasticity. Salk Institute for Biological Studies, La Jolla, United States. doi:10.7554/eLife.10778.001
- Bashwiner D., Bacon D., 2018. Musical creativity and the motor system. University of New Mexico, Albuquerque, United States. doi:10.1016/j.cobeha.2018.12.005
- Bashwiner D.M., Wertz C.J., Flores R.A., Jung R.E., 2015. Musical Creativity “Revealed” in Brain Structure: Interplay between Motor, Default Mode, and Limbic Networks. University of New Mexico, Albuquerque, United States. doi:10.1038/srep20482
- Benner J., Wengenroth M., Reinhardt J., Stippich C., Schneider P., Blatow M., 2017. Prevalence and function of Heschl’s gyrus morphotypes in musicians. University of Heidelberg Medical School, Germany. doi:10.1007/s00429-017-1419-x
- Chanda M.L., Levitin D.J., 2013. The neurochemistry of music. McGill University, Montral, Canada. doi:10.1016/j.tics.2013.02.007
- Cheung V.K.M., Meyer L., Friederici A.D., Koelsch S., 2017. The right inferior frontal gyrus processes nested non-local dependencies in music. Max Planck Institut Leipzig, Germany; University of Bergen, Norway. doi:10.1038/s41598-018-22144-9
- Christiner M., Reiterer S.M., 2015. A Mozart is not a Pavarotti: Singers outperform instrumentalists on foreign accent imitation. University of Vienna, Austria. doi:10.3389/fnhum.2015.00482
- Cohen A.J., Levitin D., Kleber B.A., 2020. Brain mechanisms underlying singing. doi:10.4324/9781315163734-6
- Corballis M.C., 2014. Left Brain, Right Brain: Facts and Fantasies. School of Psychology, University of Auckland, New Zealand. doi:10.1371/journal.pbio.1001767
- Englander Z.A., Pizoli C.E., Batrachenko A., Sun J., Worley G., Mikati M.A., Kurtzberg J., Song A.W., 2013. Diffuse reduction of white matter connectivity in cerebral palsy with specific vulnerability of long range fiber tracts. Duke University Medical Center, United States. doi:10.1016/j.nicl.2013.03.006
- Gaser C., Schlaug G., 2003. Brain structures differ between musicians and non-musicians. University Jena, Germany, Harvard Medical School, Boston United States. doi:10.1523/JNEUROSCI.23-27-09240.2003
- Halwani G.F., Loui P., Rüber T., Schlaug G., 2011. Effects of practice and experience on the arcuate fasciculus: comparing singers, instrumentalists, and non-musicians. Harvard Medical School, Boston, United States. doi:10.3389/fpsyg.2011.00156
- Hill V.B., Cankurtaran C.Z., Liu B.P., Hijaz T.A., Naidich M., Nemeth A.J., Gastala J., Krumpelman C., McComb E.N., Korutz A.W., 2019. A Practical Review of Functional MRI Anatomy of the Language and Motor Systems. Northwestern University Feinberg School of Medicine, Chicago, United States. doi:10.3174/ajnr.A6089
- Howard D.M., 2020. Synthesis of a Vocal Sound from the 3,000 year old Mummy, Nesyamun ‘True of Voice’. Royal Holloway, University of London, United Kingdom. doi:10.1038/s41598-019-56316-y
- Jürgens U., 2009.The Neural Control of Vocalization in Mammals: A Review. German Primate Center Göttingen, Germany. doi:10.1016/j.jvoice.2007.07.005
- Kaufmann C., Auer D.P., 2002. Brain imaging in psychiatry. Psychotherapie 7. Jahrg. 2002, Bd. 7, Heft 2. CIP-Medien, München
- Kleber B., Friberge A., Zeitounic A., Zatorre R., 2016. Experience-dependent modulation of right anterior insula and sensorimotor regions as a function of noise-masked auditory feedback in singers and nonsingers. Canada, Germany, Sweden, Denmark. doi:10.1016/j.neuroimage.2016.11.059.
- Kleber B., Veit R., Birbaumer N., Gruzelier J., Lotze M., 2009. The Brain of Opera Singers: Experience- Dependent Changes in Functional Activation. Germany, Italy, United Kingdom. doi:10.1093/cercor/bhp177
- Kleber B.A., Zarate J.M., 2014. The neuroscience of singing. Oxford University Press, Oxford, United Kingdom. doi:10.1093/oxfordhb/9780199660773.013.015
- Koelsch S., 2012. Brain and Music. John Wiley & Sons Ltd., Chichester, United Kingdom. ISBN: 978-0-470-68339-2
- Kolb, B., Muhammad, A., Gibb, R., 2010. Searching for factors underlying cerebral plasticity in the normal and injured brain, Journal of Communication Disorders. University of Lethbridge, Canada. doi:10.1016/j.jcomdis.2011.04.007
- Loui P., 2015. A dual-stream neuroanatomy of singing. Wesleyan University, Universitiy of California Press, United States. doi:10.1525/MP.2015.32.3.232
- Luo N., Sui J., Abro A., Turner J.A., Damara E., Fu Z., Fan L., Chen J., Lin D., Zhuo C., Xu Y., Glahn D.C., Rodrigue A.L., Banich M.T., Pearlson G.D., Calhoun V.C., 2019. Structural brain architectures match intrinsic functional networks and vary across domains: A study from 15000+ individuals. China, United States. doi:10.1101/2019.12.17.879502
- Martínez-Molina N., Mas-Herrero E., Rodríguez-Fornells A., Robert J. Zatorre R.J., Marco-Pallerés J., 2019. White matter microstructure reflects individual differences in music reward sensitivity. Universitiy of Barcelona, Spain; McGill University, Canada. doi:10.1523/JNEUROSCI.2020-18.2019
- Oikkonen J., Huang Y., Onkamo P., Ukkola-Vuoti L., Raijas P., Karma K., Vieland V.J., Järvelä I., 2015. A genome-wide linkage and association study of musical aptitude identifies loci containing genes related to inner ear development and neurocognitive functions. Univerity of Helsinki, Finland. doi:10.1038/mp.2014.8
- Oxenham A.J., 2018. How we hear: The perception and neural coding of sound. University of Minnesota, United States. doi:10.1146/annurev-psych-122216-011635
- Patel A.D., 2007. Music, language and the brain. Oxford University Press NewYork, United States. doi:10.1093/acprof:oso/9780195123753.001.0001
- Poeppel D., 2003.The analysis of speech in different temporal integration windows: Cerebral lateralization as ‘asymmetric sampling in time’. New York University, United States. doi:10.1016/S0167-6393(02)00107-3
- Segado M., Hollinger A., Thibodeau J., Penhune V., Zatorre R.J., 2018 Partially Overlapping Brain Networks for Singing and Cello Playing. Concordia University Montreal, Canada. doi:10.3389/fnins.2018.00351
- Steel C.J., Zatorre R.J., 2018. Practice makes plasticity. Max Planck Institute, Leipzig Germany; McGill University, Montreal, Canada. doi:10.1038/s41593-018-0280-4
- Toga A.W., Thompson P.M., 2003. Mapping brain asymmetry. UCLA School of Medicine, Los Angeles, United States. doi:10.1038/nrn1009
- Tan Y.T., McPherson G.E., Peretz I., Berkovic S.F., Wilson S.J., 2014. The genetic basis of music ability. University of Melburne, Australia. doi:10.3389/fpsyg.2014.00658
- Thomas G., 2013. Methoden der Kognitiven Neurowissenschaft: Kurze Einführung in die funktionelle Bildgebung, ModulA1 SS 2013. TU Dresden
- Wang W., Wie L., Chen N., Jones J.A., Gong G., Liu H., 2019 Decreased Gray-Matter Volume in Insular Cortex as a Correlate of Singers’ Enhanced Sensorimotor Control of Vocal Production. University Guangzhou; Beijing Normal University, China; Wilfrid Laurier University, Canada. doi:10.3389/fnins.2019.00815
- Zamorano A.M., Zatorre R.J., Vuust P., Friberg A., Birbaumer N., Kleber B., 2019. Enhanced insular connectivity with speech sensorimotor regions in trained singers – a resting-state fMRI study. Germany, Switzerland, Denmark, Spain, Canada, Sweden. doi:10.1101/793083
- Zhang Y., Chen G., Wen H., Lu K., Liu Z., 2017. Musical Imagery Involves Wernicke’s Area in Bilateral and Anti-Correlated Network Interactions in Musicians. Purdue University, West Lafayette, United States. doi:10.1038/s41598-017-17178-4
 Margarete M. Ertl has more than 25 years of experience as conductor and vocal coach for singers of all ages. She founded a non-corporate choir and singing school, advanced experience in inspiring people to be open-minded about new ways of making and perceiving music. She organised international choir meetings to explore foreign cultures and the choral world. She’s extremely fascinated in the arts in general and especially in science. She participated in global conducting Master classes. At an International Conference ”Community Music”, she presented a session on ”Developing a children’s concert choir based on Community Music – contradiction or confirmation?”. She attended each World Symposium on Choral Music since the one in 1999 in Rotterdam. She participated in international choir competitions and was awarded several prizes. She got a degree in voice training and education in Fulda/Germany and in choral conducting for children’s choirs and choral conducting in Wolfenbuettel/Germany. Contact: margarete.ertl@icloud.com
Margarete M. Ertl has more than 25 years of experience as conductor and vocal coach for singers of all ages. She founded a non-corporate choir and singing school, advanced experience in inspiring people to be open-minded about new ways of making and perceiving music. She organised international choir meetings to explore foreign cultures and the choral world. She’s extremely fascinated in the arts in general and especially in science. She participated in global conducting Master classes. At an International Conference ”Community Music”, she presented a session on ”Developing a children’s concert choir based on Community Music – contradiction or confirmation?”. She attended each World Symposium on Choral Music since the one in 1999 in Rotterdam. She participated in international choir competitions and was awarded several prizes. She got a degree in voice training and education in Fulda/Germany and in choral conducting for children’s choirs and choral conducting in Wolfenbuettel/Germany. Contact: margarete.ertl@icloud.com
Edited by Gillian Forlivesi Heywood, Italy/UK
[1] The communication between neurons at the synapses is referred to as synaptic plasticity. Two types can be discerned: ”functional plasticity” to strenghen or weeken existing synaptic connections through long-term potentiation (LTP) or long-term depression (LTD) and ”structural plasticity”: a change of existing synapses in morphology and organization, also the formation or elimination of synapses.